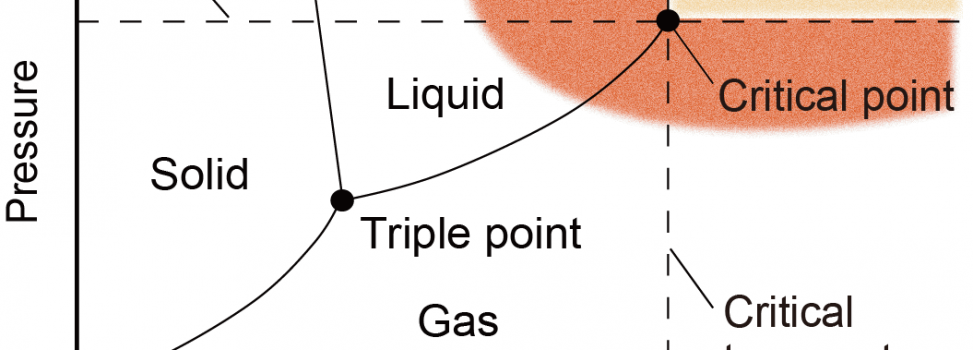
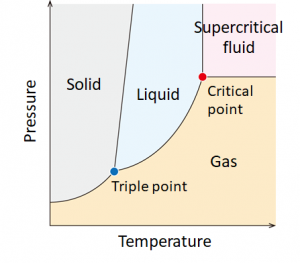
There are three states of matter; solid, liquid and gas. However, when both temperature and pressure exceed its intrinsic critical temperature (Tc) and critical pressure (Pc), the matter becomes supercritical fluid where the gas-liquid phase transition disappears. Supercritical fluid is a promising reaction field because it combines high molecular kinetic energy like a gas and high density like a liquid.
Our laboratory is studying the decomposition of biomass in various supercritical (or subcritical) fluids. For example, supercritical water can hydrolyze cellulose and hemicelluloses into saccharides without catalyst due to the high ionic product of supercritical water. In addition, supercritical fluids of various organic solvents such as alcohols can also decompose biomass and the decomposition behavior and products depend on the kind of solvent. We use various types of reactors; batch, continuous and semi-flow reactors.
What is supercritical fluid?
Supercritical fluid observed through a
sapphire glass window